iron electron configuration|longhand electron configuration for iron : Bacolod In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom (there are 26 electrons). Once we have the configuration for Fe, the ions are simple. When we write the configuration we'll put all 26 electrons in orbitals . Get the best NFL odds at BetUS! . September 2, 2024. Breaking NFL News. NFC Predictions: 49ers Head Super Bowl Power Rankings. Eagles WR’s DeVonta Smith and A.J Brown both finished within the top 20 for most receiving yards last season 49ers finished second in NFL for yards .
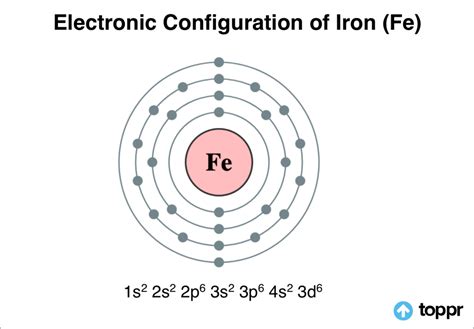
iron electron configuration,In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom (there are 26 electrons). Once we have the configuration for Fe, the ions are simple. When we write the configuration we'll put all 26 electrons in orbitals .Learn how to write the electron configuration for calcium (Ca) using the .

Learn how to write the electron configuration for sodium (Na) atom with .Learn how to write the electron configuration for nitrogen (N) using the .Learn how to write the electron configuration for potassium (K) using the .Learn how to write the electron configuration for magnesium (Mg) using .Learn how to write the electron configuration for chlorine (Cl) using the .Learn how to write the electron configuration for sulfur (S) using the .Learn how to write the electron configuration for hydrogen, the simplest .Learn the electronic configuration of iron, a transition metal with atomic number 26 and symbol Fe. Find out its oxidation states, allotropic forms, .Iron is required for life. The iron–sulfur clusters are pervasive and include nitrogenase, the enzymes responsible for biological nitrogen fixation. Iron-containing proteins participate in transport, storage and use of oxygen. Iron proteins are involved in electron transfer. Examples of iron-containing proteins in higher organisms include hemoglobin, Answer link. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. What we have is: Its core orbitals are the 1s, . The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, .The electronic configuration of Iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Electronic Configuration of Iron is - 1s2 2s2 . Learn how to determine the electron configuration of different atoms based on the principles of quantum mechanics and the periodic table. Find out the oxidation .
Using the Aufbau Principle, the Pauli Exclusion Principle, and Hund's rule to predict an atom's electron configuration using the periodic table as a guide ; Differentiate between (spdf) electron configuration, orbital box .The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .An element’s valency is the number of electrons it receives, gives away, or shares in order to achieve a noble gas or stable configuration. In layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. As previously stated, iron has two valence states: +3 and +2.The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron .
The iron electron configuration is one of the other significant areas of study for scholars of chemistry. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, the Iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 .Element Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.

In order to write the electron configuration for Iron (Fe) we first need to know the number of electrons for the Fe atom (there are 26 electrons). When we.longhand electron configuration for iron Iron is a good example. Humans have known about iron long before we knew about elements and atoms. . The electron configuration is 4s 1, 3d 10 but all these general .
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .For example, the electron configuration of iron is Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence . Iron (Fe) is a chemical element that belongs to the transition metals group in the periodic table. It is widely known for its importance in various industries, including construction, manufacturing, and medicine.The electron configuration of iron, specifically Fe2+ and Fe3+ ions, plays a crucial role in understanding its chemical properties and . The Electronic Configuration of Iron. A chemical element with an atomic number 26 and a symbol Fe represents it, Iron is a very common element that is found. Unlike other elements, iron exists at the oxidation states of -2 to +6. Elementary iron occurs in a low-oxygen environment in spite of being reactive to water and oxygen.iron electron configurationOrbital diagram. Iron electron configuration. ← Electronic configurations of elements. Fe (Iron) is an element with position number 26 in the periodic table. Located in the IV period. Melting point: 1535 ℃. Density: 7.87 g/cm 3 . Electronic configuration of the Iron atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 . We build electron configurations by filling the lowest energy orbitals first then filling progressively higher energy orbitals. This is known as the aufbau principal. . but imagine if you were .
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Iron (Fe) .
The number of electrons in the valence shell of iron (Fe) can be used to calculate the electronic configuration of the various oxidation states. Fe2+ F e 2 +: Iron now has an electron configuration of [Ar]3d6 [ A r] 3 d 6 after losing two electrons from its 4s orbital. The iron ion acquires a 2+ charge as a result.iron electron configuration longhand electron configuration for iron In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .Iron has an atomic number of 26, which means it has 26 electrons. The noble gas shorthand for Fe can be written as [Ar] 4s2 3d6. This notation indicates that the electron configuration of Fe includes the noble gas configuration of argon ( [Ar]) followed by two electrons in the 4s orbital and six electrons in the 3d orbital.
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.
iron electron configuration|longhand electron configuration for iron
PH0 · longhand electron configuration for iron
PH1 · iron electron configuration unabbreviated
PH2 · iron electron configuration diagram
PH3 · fe +2 def how many electrons added
PH4 · electron configuration calculator
PH5 · condensed electron configuration for iron
PH6 · complete the electron configuration for s
PH7 · complete electron configuration iron
PH8 · Iba pa